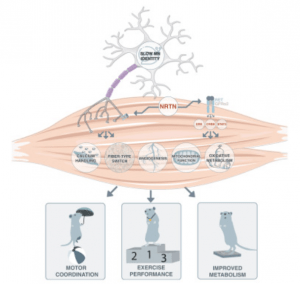
Researchers at Karolinska Institutet in Sweden have identified a protein that improves muscular metabolism, motor…Read More
This study aimed to identify the genetic variants associated with juvenile ALS. The data broadened…Read More
Topline data is expected in the next couple of months for the Clene’s Phase 2…Read More
Dropping the levels of microRNA-218 (miR-218) — a small molecule that regulates the activity of…Read More
Dosing has begun in a clinical trial of WVE-004, Wave Life Sciences‘ investigational therapy for amyotrophic lateral…Read More
Several rare disruptive gene variants have been associated with ALS and are responsible for about…Read More
This study estimated the number of prevalent and incident ALS cases overall and superoxide dismutase…Read More
University of Utah Health researchers have detected a set of genetic mutations that appear to…Read More
An international team of researchers led by scientists at the National Institutes of Health and…Read More
Orphazyme, a late-stage biopharmaceutical company pioneering the heat shock protein response for the treatment of…Read More
Biogen have announced a Two-Part Tofersen Access Program. Beginning in mid-July 2021, after patients in…Read More
This review discusses the structural and functional relevance of MAMs in ALS and…Read More
Respiratory tests are fundamental for monitoring respiratory function in ALS, and essential in clinical trials.…Read More
Neuroinflammation is an important pathogenic mechanism in amyotrophic lateral sclerosis (ALS), with regulatory T cells…Read More
Researchers from the University of Sheffield as part of the AMBRoSIA biobank, have found higher…Read More
Dr Arpan Mehta alongside Dr Bhuvaneish Selvaraj and Professor Siddharthan Chandranat Euan MacDonald Centre for…Read More
Researchers at Wake Forest School of Medicine found that ALS patients with a genetic variation…Read More
A Phase 2 clinical trial in ALS patients found that use of reldesemtiv, designed to…Read More
The FDA recently released a new guidance document to assist sponsors in the clinical development…Read More
Kadimastem Ltd., a clinical stage cell therapy company, announced promising interim results of its Phase…Read More